-40%
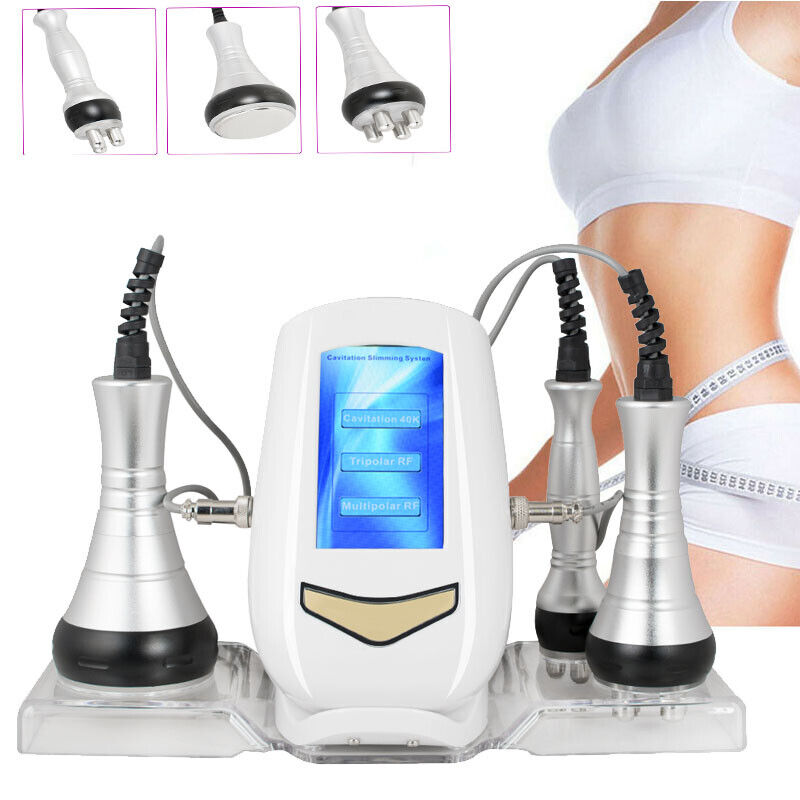
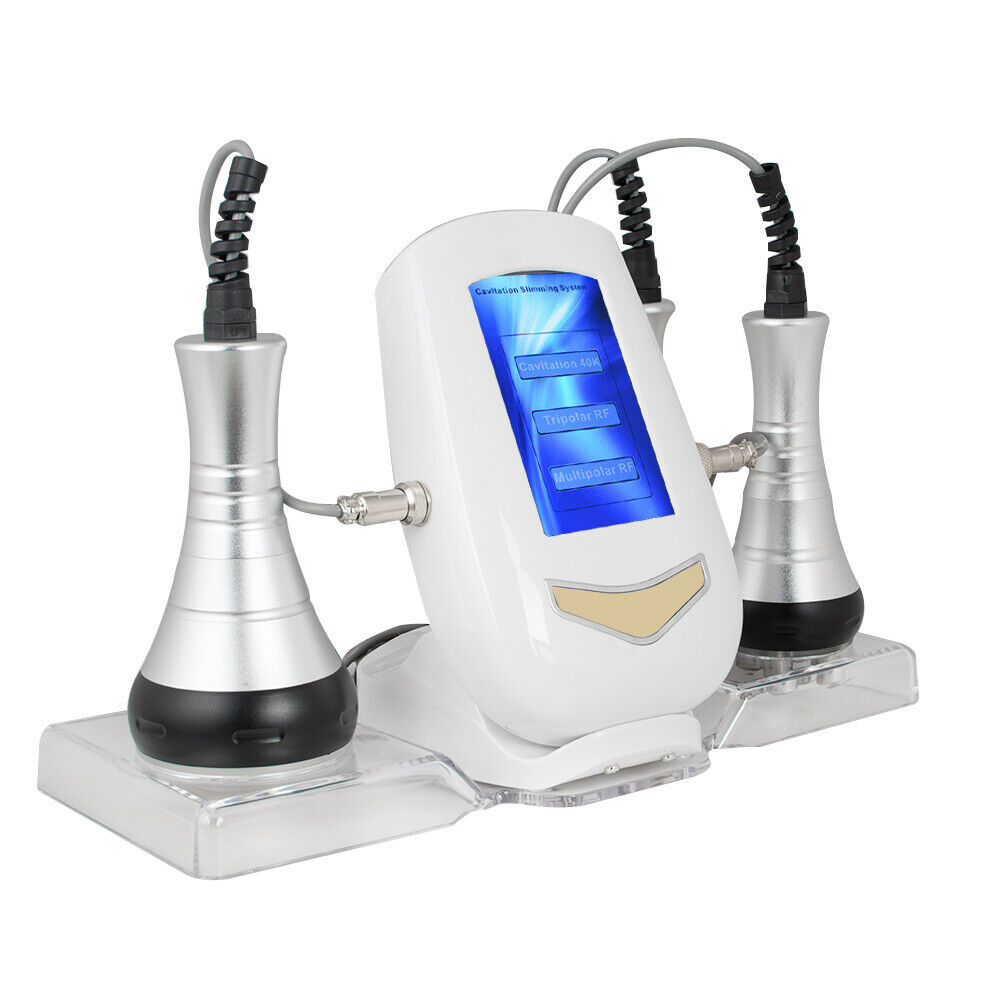
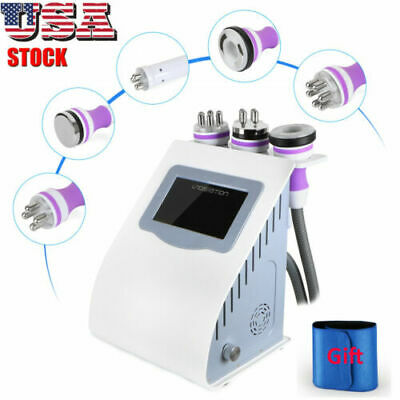
3-in-1 40K Cavitation RF Radio Frequency Vacuum Slimming Machine US
$ 60.72
- Description
- Size Guide
Description
Welcome To My ShopStore Categories
Store Categories
Dental
Curing-Light
Handpiece
Dental supplies
Dental loupes
Intraoral Camera
Burs & Bur Blocks
Polishing Micromotor
Instruments
Scaler
Sex Health
Oximeter
Scanner
Vet Use
Human Use
Endoscopy & Laparoscopy
Supplies
Patient Monitor
Vet use
Human use
Prenatal Heart Monitors
Test& Measurement Equipment
Home & Garden Accessories
Sports Accessories
Beauty Tools
Weight Loss Supplements
Skin Care
Hair Removal
Tattoos & Body Art
Eye Treatments & Masks
Hair Salon
Fetal Doppler
ECG EKG machine
Big Sale
Health Care
USA STOCK
Clearance Sale
Wholesale Deal
Business & Industrial
OFF
Allen-Bradley
Other
Product Details
Description:
Features:
1. 3-In-1 RF Radio Frequency Body Slimming Beauty Machine, with 3 functional beauty heads for the treatment of different parts of the body and guarantees its high efficacy for the treatment of various skin problems on the face and body, applicable to the face, neck, stomach, waist, thigh, calf and buttocks.
2. Provide skin oxygen and organic nutrition, enhance cell function, promote blood and lymphatic circulation, activate metabolism, remove and soften orange peel tissue, so as to achieve body slimming and cosmetic effect.
3. 40K RF beauty Machine can eliminate facial wrinkles, relieve freckles, eliminate excess fat and make skin white and smooth. will promote the metabolism, in order to restore elasticity, calm and hydrate the effects on the skin and firm skin, smooth eye wrinkles, shaded black circle, narrow pores, tighten free skin, remove double chin.
Parameters:
Product Name: 40K RF Beauty Instrument
Output power: 50W
Power input: 100V-240V
Working head: 3
Handle material: Metal
RF: 40KHz
Material: ABS
RF frequency: 5m
Screen size: 4.3 inches
Net weight: 3.5kg
Package size: 410*360*200 mm / 16.14*14.17*7.87 inches
Package Include:
1 X 40K RF Beauty Instrument
3 X
Functional Beauty Heads
1 X instructions
FDA Disclaimer
FDA Disclaimer:
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(Seller name:Edela
City:Beijing State:Beijing Country:China Phone
:+86 18332651236
)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892
Payment Method
Shipping Policy
Returns Policy
About US
Payment Method
We only accept payment via PayPal.
Payment Method
Shipping Policy
1.US Stock items Shipping Service UPS 3-5 BUSINESS DAYS (weekend and festival not included) from our warehouse in the US,
only to United-States. China Stock items will be shipped by epacket
or Airmail post within 1 business days.
2.We are not Responsible for your Customs duty.
3. We don't add taxes, VAT or other hidden charges. You pay us what you see on the invoice, i.e. goods subtotal + shipping cost (not include duties). Please find out as much as you can about your import taxes in your own country before bidding the item. You need to pay for the import duties in case that occurred for certain goods.
4.Items will be shipped to your eBay address. Please make sure it is correct.
Returns Policy
1.If you receive a defective item which you want to return, please contact us within 2 days from the day you receive the item.
2.All return items must be returned with its original packaging and accessories. If we confirm the damage happened during the shipment, we will bear the shipping cost for return.
3.We will refund the money to you when we get the return items. Or replace item for you free of charge.
About US
Our working time: Monday - Friday 9:00 AM - 6:00PM (GMT+8) .
We ship the package from Monday to Saturdays.
FDA Disclaimer:
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(Seller name:Edela City:Beijing State:Beijing Country:China Phone:+86 18332651236)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
, Treatment Device is certified with the US FDA 510(k) Number:K161892
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892
FDA Disclaimer:
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(Seller name:Edela City:Beijing State:Beijing Country:China Phone:+86 18332651236)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
,Ultrasonic Treatment Device is certified with the US FDA 510(k) Number:K161892
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892
FDA Disclaimer:
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(Seller name:Edela City:Beijing State:Beijing Country:China Phone:+86 18332651236)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
Ultrasound ,Ultrasonic Treatment Device is certified with the US FDA 510(k) Number:K161892
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892